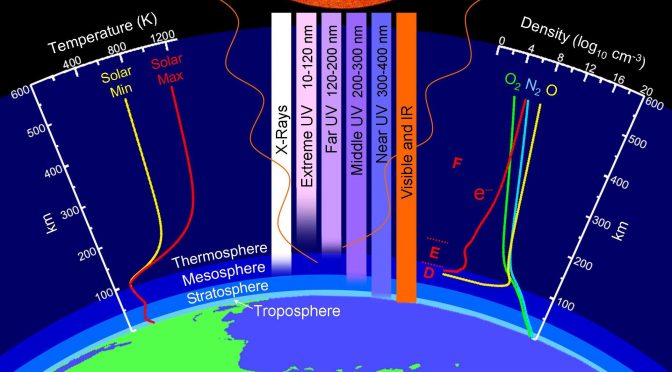
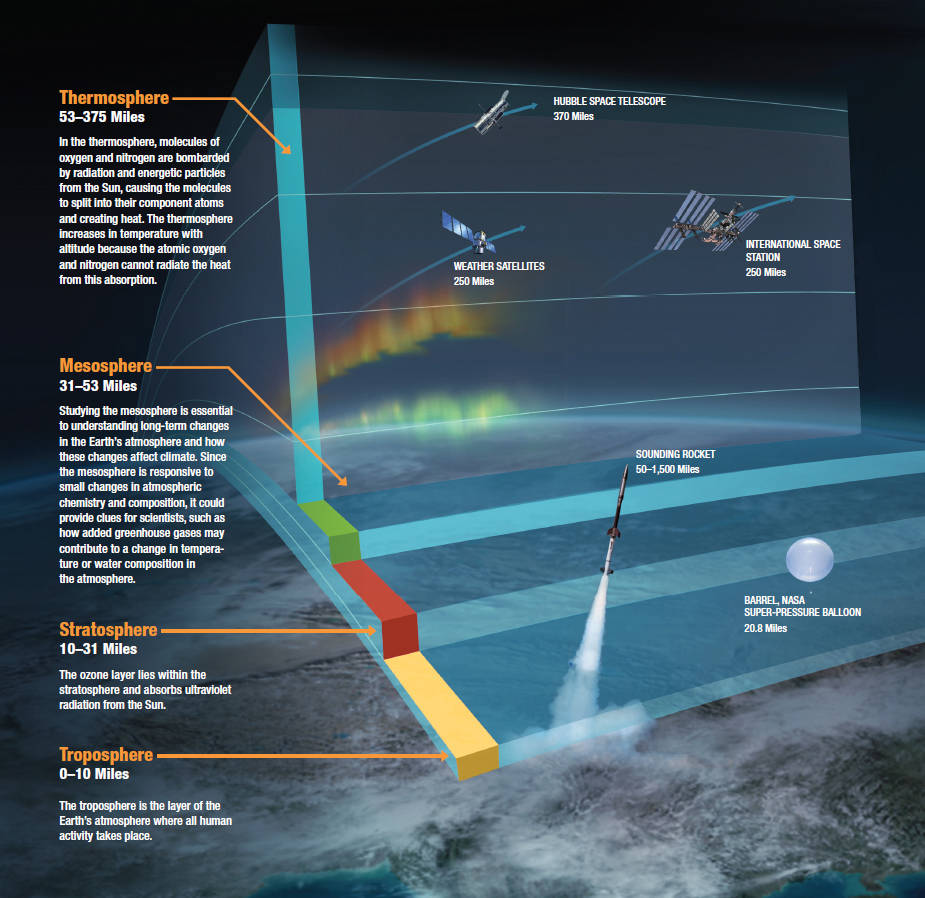
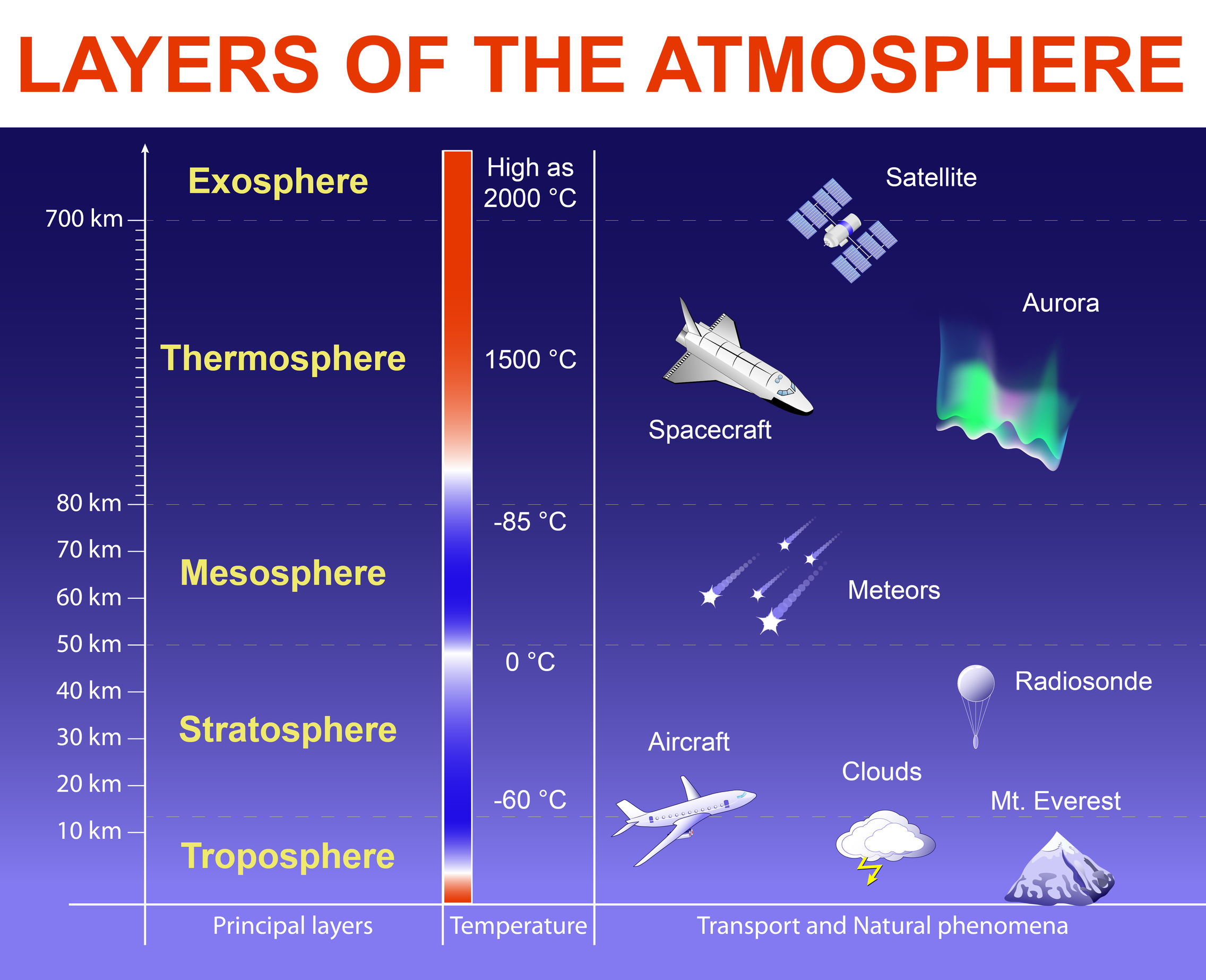
Atmospheric composition and vertical structure of the atmosphere – spatial and temporal aspects
This unit introduces you to the medium that this course focuses on: The atmosphere of the Earth.
Learning goals
After completion of this unit, students will be able to
- Explain the vertical structure of the atmosphere under the aspects of temperature, composition, pressure, ionization, escape velocity, and magnetism and how these different aspects are related to each other
- Identify the processes that lead to the observed vertical structure of Earth’s atmosphere
- Recall horizontal and vertical distributions of atmospheric constituents
- Name sources and sinks of atmospheric constituents
Students’ tasks
Note that throughout this class student’s task differ for ATM401, ATM601 and CHEM601. Thus, make sure to perform the tasks required at the level at which you take Introduction to Atmospheric Sciences.
- Watch this video that summarizes the material (all students)
- Read chapter 1.1 and 1.3 to 1.4 (included) of Lectures in Meteorology that discus the atmospheric composition and vertical structure (all students)
- If you are taking this class as
- ATM401 take these guided notes and answer the questions. Please use the book for help, but don’t copy/paste from the e-version. The notes should be understandable for you for quick review of a unit in preparation of the exams. Taking notes also serves to improve your communication skills. This guided notes part is pass/fail and part of the homework grade. Failure to submit the notes in time will lead to an F. The guided notes and the knowledge and comprehension questions will be graded according to the percentage of completions over the semester as part of the homework grade. I read them to analyze where the class had difficulties to address them. The last question serves to seek clarification from me. When you have no questions, type “N/A”. Your grade will not suffer from asking questions here! On the contrary, it is more likely to suffer when you don’t put them forward as you will miss out your chance to seek for clarification. Note that these grading policies apply to all questionnaires in this class.
- ATM601 or CHEM601 write a brief (less than 500 words, more than 200 are o.k.) summary of the key material to serve as your reading notes and copy it into this Knowledge and Comprehension form at the foreseen place. Note I will not accept any/copy paste from the book. Answer the questions. This part is pass/fail and part of the homework grade. Failure to submit the notes in time will lead to an F. It will be graded according to the percentage of completions over the semester as part of the homework grade. I read these parts to analyze where the class had difficulties to address them. The last question of the questionnaire serves to seek clarification from me. When you have no questions, type “N/A”. Your grade will not suffer from asking questions here. On the contrary, it is more likely to suffer when you don’t put them forward as you will miss out your chance to seek for clarification. Note that these grading policies apply to all knowledge and comprehension forms of the class.
- All students: Go to this unit1 task sheet and work the problems that apply to the class level you take. This means when you take the class as ATM401, only the problems indicated for ATM401 and “all students” are mandatory for you. When you take the class at ATM601 or CHEM601 only the problems indicated as such and for all students are mandatory. You can try to solve them now, but today my point is to show you how I want you to solve problems. Watch this movie that provides the solutions of problem 1 and explains how to get there .
Once you have watched the video, download the unit 1 problem solutions. Please go thru the solutions so you know how to organize your problem solutions in the future. Note you won’t have to type your solutions, nice legible hand writing is sufficient. The examples just should give you an idea of what I mean with “show how you got to the solution”.
- Participate in the discussion channel of this unit on the discussion board
- All students: Take this quiz. You are allowed to take your notes, re-watch the movie, and/or re-read sections in the book for help when taking the quiz. Please check the material again, when you are in doubt. The purpose of the quiz is to ensure that you recall the vertical structure, the horizontal distribution, sources and sinks of constituents. The above learning objectives are critical for discussion of atmospheric thermodynamics, dynamics, radiation and chemistry. Revisit the facts to remember them. In the quiz, you also will combine them and put them into a new context. The quiz will be graded by percentage of correct completion. Note that these rules apply to all future online quizzes. For your information there will be a quiz in the last unit of each chapter. This means there will be six quizzes in total.
Test your knowledge and comprehension (optional)
Additional material for the interested student (optional)
More information on the aurora.
FAQ
Q: Is water vapor the only major constituent difference between troposphere and stratosphere?
A: No. The concentrations of ozone, for instance, are much higher in the stratosphere than in the troposphere.
Q: Is there a notable difference in stratospheric ozone concentrations between the North Pole and South Pole? If yes, would it mainly due to dynamical processes or chemical processes?
A: In the respective hemispheric spring, stratospheric ozone concentrations go down. Once the Sun comes back in spring, photolysis and photo-chemistry start. Typically, spring ozone concentrations are lower at the South Pole as the cold high plateau and the quasi-circular circulation in the Antarctic cut off the exchange with the mid-latitudes. The high albedo of snow may increase photolysis rates near the surface. Thus, the high plateau on the South Pole means that this process sets on comparatively earlier than at the North Pole.
Q: The equation for probable escape velocity – it has two mass terms, M, and m. Is this a typo?
A: Yes. The probable escape velocity depends on the mass of the molecule.
Q: What is the difference between main gases of the dry atmosphere, trace gases and greenhouse gases?
A: The main gases of the dry atmosphere are N2, O2, Ar, and CO2. Trace gases are all gases that contribute with far less than ppt to the total composition, but may locally have much higher concentrations. Greenhouse gases absorb solar radiation, for instance, CO2, H2O vapor, or CH4. They can be a trace gas, e.g. CH4, but must not be a trace gas, e.g. CO2.
Q: Do all planets have the same escape velocity?
A: No. See animation.

Q: Why is the atmosphere below 100 km called the “homogenous” atmosphere when it is not actually a homogeneous system (since pressure changes with height, state variables are changing, so its not strictly homogeneous)?
© 2019 Nicole Mölders | All rights reserved