Troposhperic and Stratospheric Gasphase Chemistry
This unit covers the gas phase chemistry of the clean and polluted troposphere and connects the sources of atmospheric constituents (unit 1) in the big picture of the species’ chemical fate. It also explains the stratospheric ozone and temperature distribution we learned about in unit 1 and puts them in the context of radiation, thermodynamics of irreversible processes and stratospheric chemistry.
Learning goal
Determination of tropospheric air quality with respect to trace gases and stratospheric composition via chemical equations.
After successful completion of this unit students will be able to
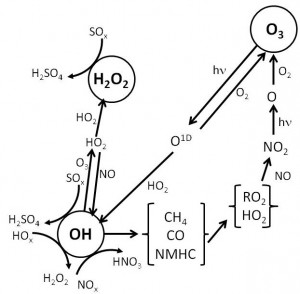
- Explain the gas phase chemistry of the clean and polluted troposphere
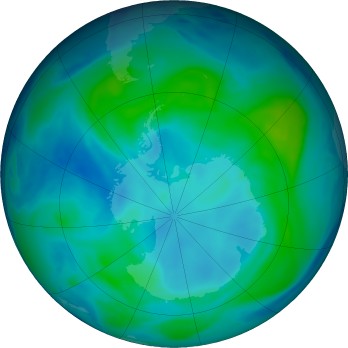
- Analyze the Chapman cycle and explain the ozone hole
- Produce and solve equations of gas phase chemistry
Students’ Tasks
- Watch this video of the material
- Read chapters 5.2.5 to 5.2.6.2 (included) of Lectures in Meteorology and take notes.
- Watch this video of an example worked problem on the triad-NO-NO2-O3. It combines aspects from this unit, unit 14 and concepts we covered when discussing thermodynamics and radiation.
- Watch this video of a worked problem
- All students: Participate in the discussion channel of this unit on the discussion board
- Please answer the questionnaire.
- Solve the problems assigned at your class level in this unit15 work sheet and submit the scanned solution file with your name by Thursday 2359 Alaska time.
Supplemental Material
You can find the powerpoint presentation here.
The video below shows a worked problem example related to the Chapman cycle.
This video shows on how to balance equations
Cool Stuff
Watch movies of the ozone evolution in the Southern Hemisphere
or Northern Hemisphere
FAQ
Q: How do I convert concentrations into other units?
A: Watch this video for a how-to.
© 2019 Nicole Mölders | All rights reserved