Aqueous phase chemistry
In Chapters 1, 2 and 3, we introduced aerosols and cloud condensation nuclei and their role for cloud formation. In this unit, we will build on these concepts by introducing the the concepts of solution, gas transfer into drops, ionization and reactions within the solution.
Learning goal
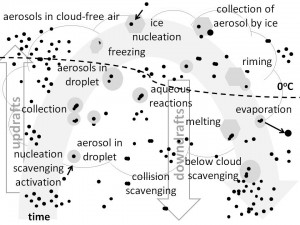
Description, quantification and discussion of chemical processes within cloud and rain drops.
After successful completion of this unit students should be able to
- Apply Henry’s law to determine dissolution of soluble trace gases
- Determine ionization
- Construct aqueous phase reaction chains and the temporal evolution of concentrations in droplets
- Determine pH values
- Analyze heterogeneous chemistry processes
- Write gaseous-aqueous phase equilibrium, ionization, and aqueous phase reaction equations as well as dissolution rate, and ionization rate equations.
- Assess impacts of atmospheric trace gases on water of various systems (graduate students)
Students’ Tasks
- Prior to watching the video, review the section Raoult’s Law, and the sections on CCNs, diffusion, enthalpy and phase transitions as well as Dalton’s law as these concepts are fundamental for understanding of aqueous chemistry
- Watch this video on the material
- Participate in the discussion channel of this unit on the discussion board
- Read chapter 5.3 to 5.3.4.6 (included) of Lectures in Meteorology
- Watch this movie that shows an example of a worked problem on how to determine changes between the gas phase and aqueous phase.
- Participate in the discussion channel of this unit on the discussion board
- Answer the questionnaire by the deadline
- Solve the problems assigned at your class level in this unit16 work sheet by Thursday 2359 AKST and email them to cmoelders@alaska.edu.
Supplemental Material
You can find the powerpoint presentation of this unit here.
FAQ
Q: What is the difference between Sulfur IV and Sulfur VI, and why should we care about it?
A: This video explains the difference and why we care about the distinction
Q: I have difficulty to grabs the idea behind S(IV) and S(VI). What is S(IV) and S(VI)?
A: This video explains the concepts of S(IV) and S(VI).
Q: How do I deal with the reaction rates?
A: This video explains how to collect the reaction rates.
© 2019 Nicole Mölders | All rights reserved

