Aerosols and biogeochemical cycles
So far we addressed gaseous and liquid species in the atmosphere. However, some gases can exist in their gas phase or solid phase in the atmosphere depending on their concentration and temperature. We already talked about particles and their role in lowering the saturation pressure within the framework of nucleation as well as their role in the radiation transfer. This unit further elaborates the role of atmospheric particles in the thermodynamics of irreversible processes and the Earth system.
Learning goal
Upon successful finishing this unit students can
- explain the basics of aerosol formation and growth from precursor gases
- interpret aerosol size distributions
- explain atmospheric aerosol removal processes in the framework of thermodynamics, cloud microphysical, and chemical processes (graduate students)
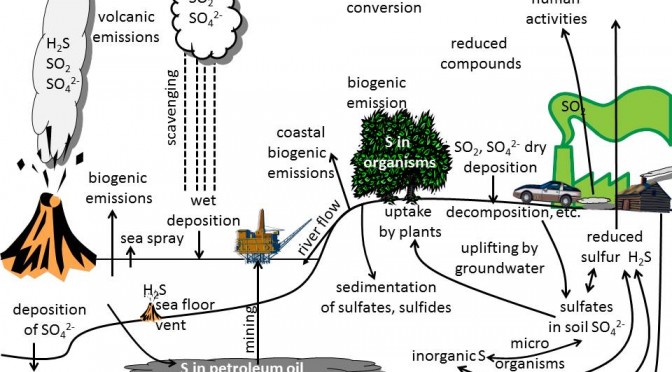
- biogeochemical cycles of the Earth’s system, and identify linkages (graduate students)
Students’ Tasks
- Repeat the sections on sinks and sources of atmospheric constituents (Chap. 1), the gas laws, moisture measure, saturation (Chapt. 2), heterogenous nucleation, diffusion (Chap. 3), absorption and scattering (Chap. 4) as they are central to understanding of the linkages of the biogeochemical cycles.
- Watch this video
- All students: Read chapter 5.4 with all its subsections of Lectures in Meteorology
- Graduate students: Read chapter 5.5 to 5.6.3 (included) of Lectures in Meteorology
- Answer the questionnaire
- Participate in the discussion channel of this unit on the discussion board
- Watch this movie of a worked problem
Supplemental Material
You can find the powerpoint presentation here.
FAQ
Q: What’s the Arrhenius equation about?
A: Watch this video.
Q: For a given aerosol particle, how can we judge whether it is formed through gas-to-particle conversion or it is formed via direct emission?
A: The size may give hints as new particles are in the Aitken nucleus range. Sulfur, nitrogen, organic and carbonaceous materials are the major chemical species involved in gas-to-particle-conversion. This size range is the nucleation mode. Here, most particles formed by oxidation of sulfur containing precursor gases like SO2, H2S, CS2, COS, CH3SCH3, or CH3SCH3 to sulfate (SO4=), and subsequent condensation, a process called homogenous gas-to-particle conversion. Many of these sulfate aerosols finally end up in the 0.1-1 micrometer size range. Sulfur dioxide, for instance, can form various sulfates in the presence of water and ammonia (NH3) by gas-to-particle conversion.
Q: How do thermophoretic processes look like when at work?
A: Watch this animation
© 2019 Nicole Mölders | All rights reserved