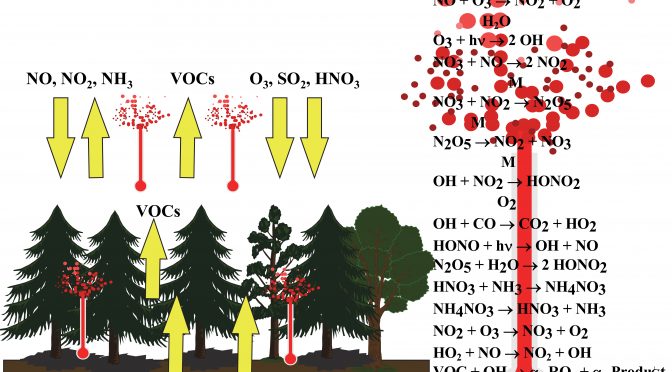
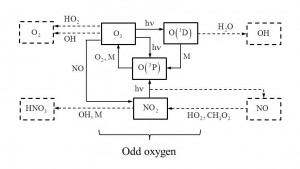
Basic concepts of atmospheric chemistry
In the discussion of the first and second laws of thermodynamics, we left discussing the chemical potential out. We also left out discussing the chemical aspects of thermodynamics of irreversible processes. The book chapter that we start to work on now, will introduce us the atmospheric chemistry and biogeochemical cycles and hydrochemical processes which are important the interactions between the water, energy and trace species cycles, and for the Earth-system at-large.
Learning goal
Discuss the fate of gases in terms of lifetime and chemical equations.
After this unit students can
- Explain the basic terminology of atmospheric chemistry
- Balance, produce and solve chemical reaction equations
- Explain the link between radiation and atmospheric chemistry, photolysis and absorption
- Calculate concentrations
- Apply the thermodynamic concepts of enthalpy, Gibbs free energy and radiation to solve atmospheric chemistry problems (Graduate students)
Students’ Tasks
-
- Review the the sections on enthalpy and Gibbs free energy in the thermodynamics and the sections on absorption in the atmospheric radiation chapter. Be aware that this unit builds on knowing and understanding those concepts.
- Watch this video on the material
- Read chapters 5. to 5.2.4.1 (included) of Lectures in Meteorology and take notes.
- Watch this video of a worked problem on NO2 photolysis and radiation in the video below.
- Create an equation and constant sheet. Hint: Among others, put the “recipe” formulas on your equation sheet.
- Watch this video of a worked problem on NO2 dissociation.
- Participate in the discussion channel of this unit on the discussion board
- Answer the questionnaire
- Solve the task assigned at your class level in this unit14 work sheet and send your solutions to cmoelders@alaska.edu by Thursday 2359 Alaska time.
Supplemental material
You can find the unit14 powerpoint presentation used in the movie at the link.
FAQ
Q: How do I balance equations?
A: This video shows on how to balance equations
© 2019 Nicole Mölders | All rights reserved