Application of gas laws in atmospheric sciences
This unit provides first tools to describe the atmosphere by means of gas laws, and the vertical profiles of temperature, density, and pressure in a quantitative way, as well as introduces the concept of gravitational force and buoyancy for vertical motions in the atmosphere.
Learning goal
After this unit, students will be able to
- Apply the gas laws that they already know from physics and/or physical-chemistry to atmospheric sciences
- Apply Archimedes law to an atmospheric column to derive the hydrostatic equation
- Evaluate the isothermal atmosphere, homogeneous atmosphere, polytrop atmosphere and US standard atmosphere
- Apply the derived equations for these atmospheres to solve typical problems
- Make assumptions on the atmospheric profile and discuss the consequences there off, the limited validity and error (graduate students only)
Students’ Tasks
- All students: Watch this video on the material
It summarizes applying the gas and Archimedes’ laws to the atmosphere and discusses the relation between buoyancy, the ideal gas law, and gravitation in the special cases of the isothermal, homogeneous, and polytrop atmosphere, and the US standard atmosphere.
- All students: Read chapter 2. to 2.3.4 (included) of Lectures in Meteorology. This reading discusses the relation between buoyancy, the ideal gas law, and gravitation. It explains the special cases of the isothermal, homogeneous, and polytrop atmosphere, and the US standard atmosphere, which provide helpful tools for evaluation of the thickness of atmospheric layers, and construction of surface weather maps needed for weather forecast.
- ATM401: Fill out the questionnaire and submit it by Thursday 2359 AST. Part 1 of the questionnaire is P/F and serves to take notes. Part 2 is graded for percentage of correct completion and measures your comprehension of the material. Failure of submission in time leads to an F. Part 3 serves to seek clarification and is not graded. ATM601/CHEM601: Write a brief summary of the read material, submit your summary and answer the question”What concept did you find particularly difficult to understand or do you need clarification on?” by Thursday 2359 AST. The summary measures your comprehension of the material. Failure of submission in time leads to an F. The question serves to seek clarification and is not graded.
- All students: Watch this video of a worked problem
- All students: Participate in the discussion channel of this unit on the discussion board
- All students: Download this unit 2 task sheet with applications of the gas laws and equations of the isothermal, homogeneous, and polytrop atmospheres. Solve the problems that apply to the class you take. This means when you take the class as ATM401, only the problems indicated for ATM401 and “all students” are mandatory for you. When you take the class at ATM601 or CHEM601 only the problems indicated as such and for all students are mandatory. Your solutions should clearly show how you got to your results. Once you are done with the tasks, scan them in and save them as one PDF file with your name and the unit indicated. If I, for instance, would submit my solutions, the name of the PDF-file would be Nicole-Mölders-unit-2.pdf. If you worked in a group of 3 students the name would be student1_student2_student3_unit2.pdf where student1, student2, and student3 are the names of the students in the group. Submit your scanned in PDF solutions file to cmoelders@alaska.edu. Don’t know how the create a pdf-file? Go to the FAQ for help.
Supplementary materials
You can find the powerpoint used in the video here, in case you want to take your notes on the slides.
Student Self Assessment of Learning Outcome (optional)
Take this quiz to self-assess your knowledge and comprehension. The assigned points add up to 100. Thus, 70 points, for instance, mean you got 70% right. You can find how percentage converts to letter grades in the syllabus.
FAQ
Q: Extensive, intensive quantities, what the heck is the difference and why should I worry about them?
A: Watch this video for an explanation, why you have to care and why it is damned important to distinguish between them
Q: Where do we apply the hypsometric equation?
A: To assess the thickness of the layer between two pressure surface for synoptic purposes.
Q: Is the individual gas constant of moist air and the same as the individual gas constant of water vapor?
No. The individual gas constant of water vapor is 461 J/(kg K) and relates to the water vapor as the only gas. On the contrary, the individual gas constant of moist air relates to a mixture of gases that among other species includes a certain fraction of water vapor. The individual gas constant of moist air has always to be calculated depending on the fraction of water vapor that the moist air holds.
Q: How do I get from Dalton’s Law to the solution in the first example of page 32 in Lectures in Meteorology?
A: Watch this video for an explanation
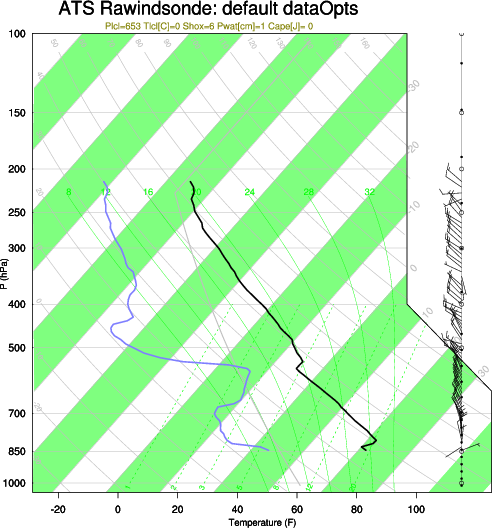
Q:What’s the difference between the US standard atmosphere and a polytrop atmosphere?
A: The US standard atmosphere is a hypothetical vertical distribution of atmospheric temperature, pressure, and density that serves as a representative atmosphere for pressure altimeter calibrations, ballistic tables, design of aircraft and missiles, aircraft performance calculations and alike. For more information see the AMS glossary. A polytrop atmosphere is a real atmosphere. For a comparison see the diagram below.
© 2019 Nicole Mölders | All rights reserved